

For reasons of structural complexity, a single condensed chemical formula (or semi-structural formula) may correspond to different molecules, known as isomers. Since a chemical formula must be expressed as a single line of chemical element symbols, it often cannot be as informative as a true structural formula, which is a graphical representation of the spatial relationship between atoms in chemical compounds (see for example the figure for butane structural and chemical formulae, at right). However, even a condensed chemical formula is necessarily limited in its ability to show complex bonding relationships between atoms, especially atoms that have bonds to four or more different substituents. An example is the condensed molecular/chemical formula for ethanol, which is CH 3−CH 2−OH or CH 3CH 2OH. This is possible if the relevant bonding is easy to show in one dimension.
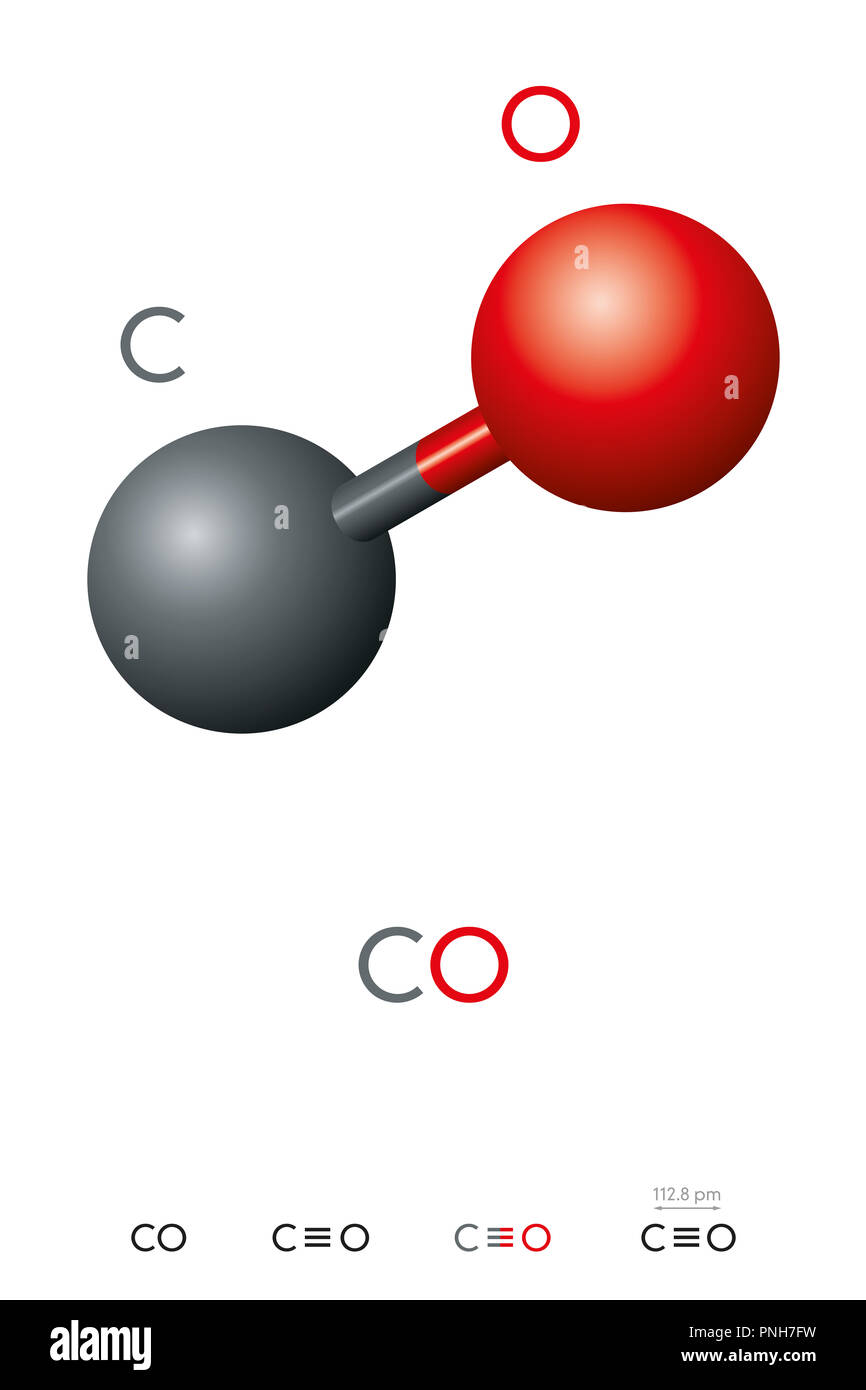
Sometimes a chemical formula is complicated by being written as a condensed formula (or condensed molecular formula, occasionally called a "semi-structural formula"), which conveys additional information about the particular ways in which the atoms are chemically bonded together, either in covalent bonds, ionic bonds, or various combinations of these types. For example, the empirical formula for glucose is CH 2O (twice as many hydrogen atoms as carbon and oxygen), while its molecular formula is C 6H 12O 6 (12 hydrogen atoms, six carbon and oxygen atoms). Molecular formulae indicate the simple numbers of each type of atom in a molecule, with no information on structure. The simplest types of chemical formulae are called empirical formulae, which use letters and numbers indicating the numerical proportions of atoms of each type. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.
#Co element formula full
Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. A chemical formula is not a chemical name since it does not contain any words. These are limited to a single typographic line of symbols, which may include subscripts and superscripts.
#Co element formula plus
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. Putting these pieces together gives the name carbon tetrachloride for this compound.Al 2 ( SO 4 ) 3 Structural formula for butane The second element, chlor ine, becomes chlor ide, and we attach the correct numerical prefix (“tetra-”) to indicate that the molecule contains four chlorine atoms. The name begins with the name of the first element-carbon. Let us practice by naming the compound whose molecular formula is CCl 4. *This prefix is not used for the first element’s name. \): Numerical Prefixes for Naming Binary Covalent Compounds Number of Atoms in Compound


 0 kommentar(er)
0 kommentar(er)
